Oilfield Water: The Hidden Companion of Oil and Gas
The Distribution of Oil, Gas, and Water
Most people are familiar with the water flowing in rivers, lakes, and seas, and have some understanding of the extraction and use of shallow groundwater. However, few are likely aware of what oilfield water is. In oilfields, oil, gas, and water are stored together in underground rock formations, and the groundwater found there is commonly referred to as oilfield water. So, how are oil, gas, and water distributed within an oilfield?
In an underground oil reservoir, gravitational differentiation causes gas, due to its low density, to occupy the top layer. Oil, being less dense than water but denser than gas, settles in the middle, while water, being the heaviest, naturally sinks to the bottom.
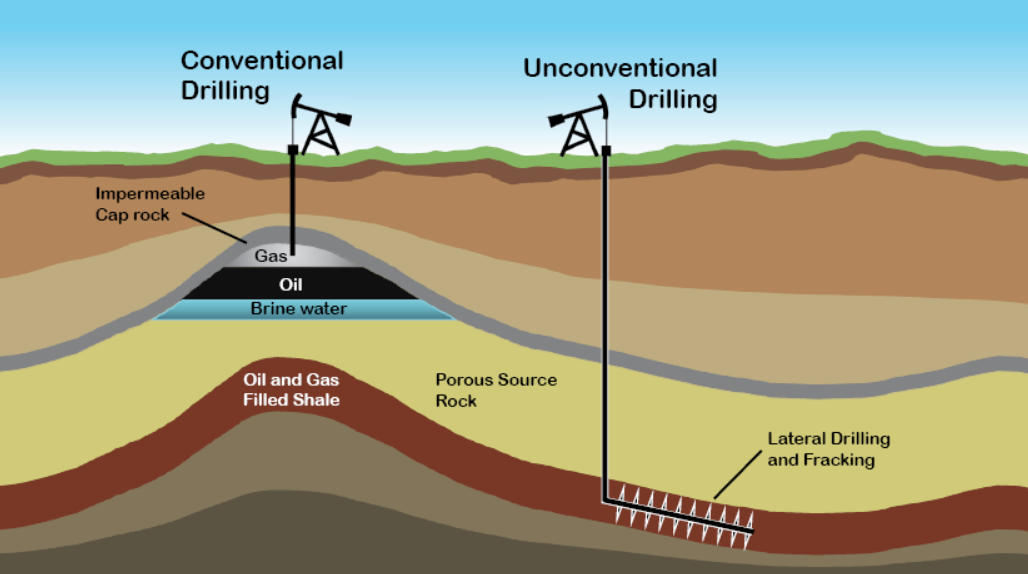
Formation of Oilfield
Oilfield water significantly differs from the shallow groundwater extracted from water wells, as its origins are far more complex. In addition to some infiltrated atmospheric water, oilfield water primarily consists of sedimentary water trapped during the deposition of rock layers, as well as deep-seated water originating from the Earth's crust. As sedimentary organic matter generates hydrocarbons, it also produces a large amount of water, making it a close companion to oil and gas. These various types of groundwater mix together to form what we call oilfield water.
A Distinct Chemical Makeup
Over long geological periods, oilfield water undergoes a series of physical, chemical, and biochemical processes that continuously alter its ionic composition. For instance, sulfates are reduced into odorous hydrogen sulfide, carbonate ions (HCO3⁻, CO3²-) increase significantly, and iron is largely oxidized into FeSO4. Due to metamorphism and high concentration levels, oilfield water contains abundant minerals, meaning it has high salinity. Over extensive geological time, it continuously dissolves soluble minerals from surrounding rocks, particularly from formations like evaporites and carbonates. As a result, oilfield water commonly contains ions such as Na⁺, K⁺, Ca²⁺, Mg²⁺, Cl⁻, SO₄²⁻, HCO₃⁻, and CO₃²⁻, which can be alkaline or acidic. It also often contains organic compounds like hydrocarbons, phenols, and organic acids, generally classifying it as "hard water."
The types of oilfield water vary across different regions. Based on the ratios of various ions in the formation water within oilfield rock layers, scientists categorize oilfield water into four types: sodium sulfate (Na2SO4) type, sodium bicarbonate (NaHCO3) type, magnesium chloride (MgCl2) type, and calcium chloride (CaCl2) type. In areas where oilfields are distributed, the calcium chloride type is the most common, followed by the sodium bicarbonate type. Regions with sodium sulfate type water generally show no oil and gas distribution. This correlation between oilfield water type and the distribution of oil and gas fields is utilized during early exploration. By analyzing water types in various basins, scientists can identify areas where groundwater is stagnant versus areas with active water exchange, helping to pinpoint regions with high potential for oil and gas accumulation. Unsurprisingly, areas with active water exchange are unfavorable for the preservation of oil and natural gas.
Once an oil and gas field is developed, water is often injected into the reservoir to enhance oil and gas recovery. Scientists add specific minerals to this injected water as "tracers," helping petroleum engineers understand the movement of groundwater within oil layers and thereby improving recovery efficiency.
The Challenge of Produced Water
Formation water and injected water together constitute the main sources of produced water in oilfield production. This type of wastewater has the following characteristics:
•High salinity: accelerates corrosion and complicates biochemical wastewater treatment.
•High oil content: far exceeding the water quality standards required for various disposal methods.
•Abundant microorganisms: whose proliferation can corrode pipelines and cause severe formation blockages.
•High concentrations of scale-forming ions: such as SO₄²⁻, CO₃²⁻, Ca²⁺, Mg²⁺, and Ba²⁺.
•Elevated levels of suspended solids: (e.g., polymers in polymer-flooding areas), which are fine and prone to causing formation blockages.
